Recent Posts
Basic Introduction of Equipment Development & Qualification (Part 2)
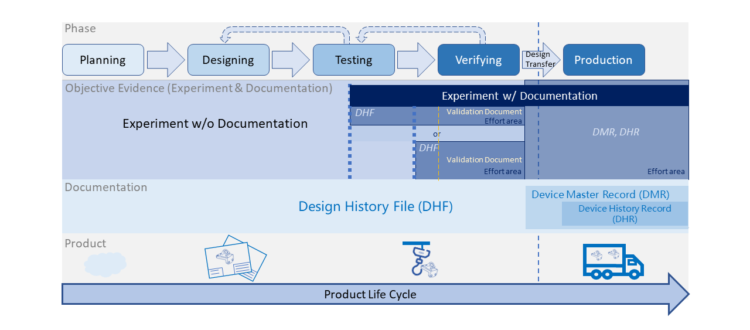
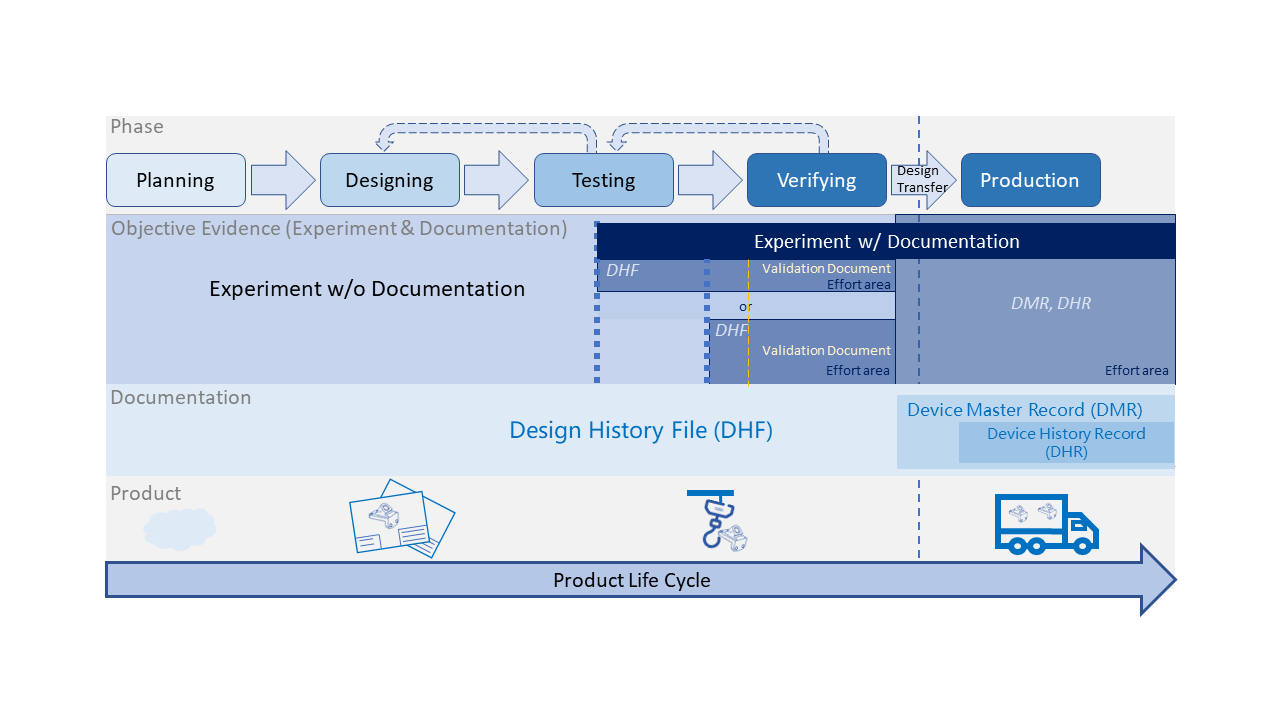
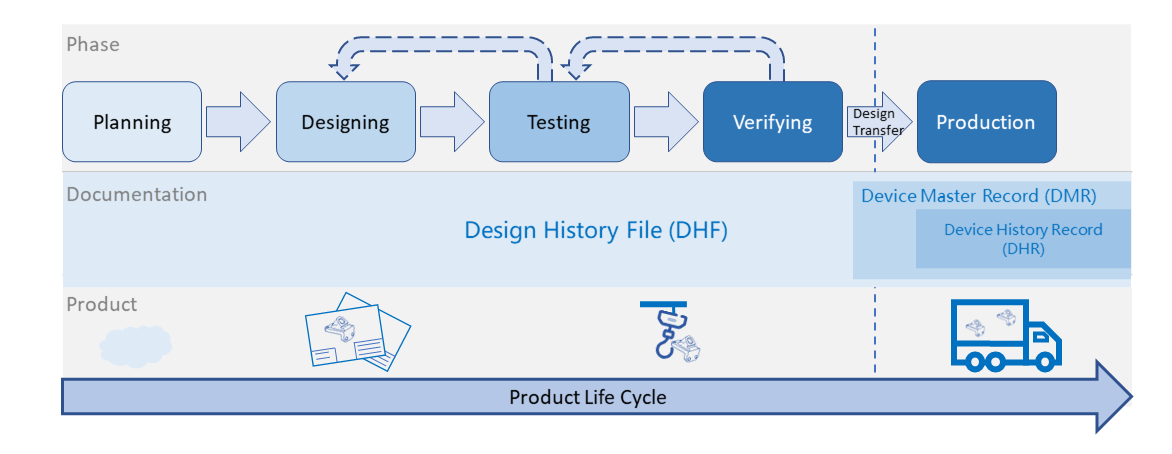
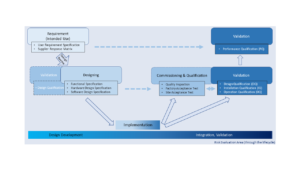
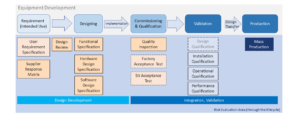
From last section -Basic Introduction of Equipment Development & Qualification, we have a general structure about equipment development... Read More
Basic Introduction of Equipment Development & Qualification
The objective of validation is to provide the evidence that the process can consistently and reproducibly produce parts... Read More
Categories
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| 28 | 29 | 30 | 31 | |||