Medical Device- Design Control Documentation (DHF, DMR, DHR)
To launch a new product in medical device industry, many documents (e.g. design documents, verification documents, controlled documents, production records) are required to prove the product’s functionality and manufacturing process capability. It easily understands that the product quality shall be controlled and the well-known evidence of control method is documentation. These documents are required as part of “Design Control” written in regulation.
There are 3 terminologies used in medical device industry to categorize different phases and scope of the product design in quality system. That is, Design History File (DHF), Device Master Record (DMR), and Device History Record (DHR).
Here under is the definition of these words defined by Food and Drug Administration (FDA) with 21 CFR Part 820
Compilation of records which describes the design history of a finished device.
Compilation of records containing the procedures and specifications for a finished device.
Compilation of records containing the production history of a finished device.
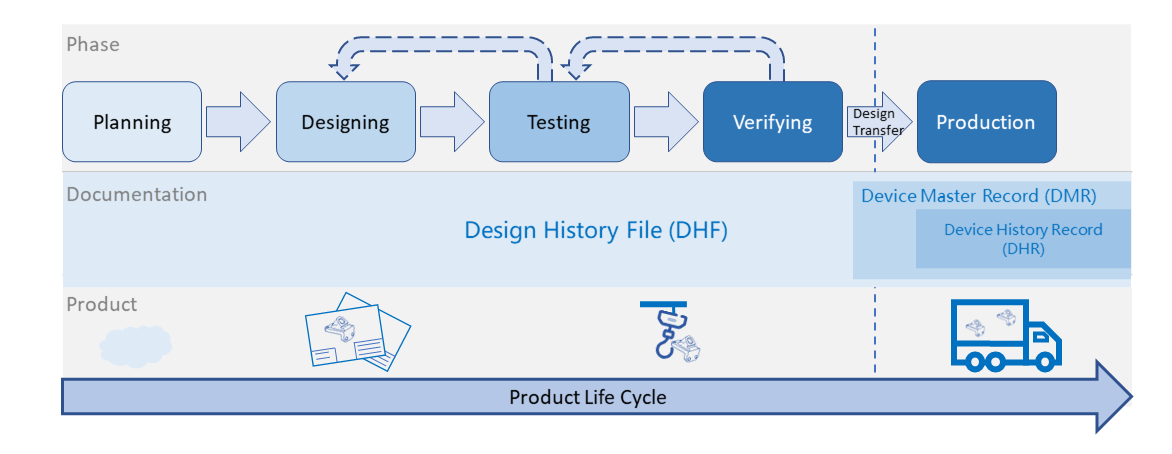
The relationship of these 3 terminologies could be explained via Figure 1 below.
From the product life cycle point of view, medical device development could be divided to “before” and “after” production phases.
DHF is a major part before production which is the record to verify the product from none to reality. Many experiments are conducted and tests results are recorded before production to prove the design is suitable for intended use. If any unacceptable result happens, the product design shall be revised and back to the previous step until all test results are acceptable and documentation.
DMR is a documentation package sourced from DHF records and required before Design Transfer which is a transition period from development to production phase. These documents could be considered as a guidance for product manufacturing process with clear definition of operating procedure and specification. While following the DMR documents, product can be consistently produced and the product quality is considered under controlled.
DHR is the production record of each production batch. Production line shall record the procedure and specification of each production batch which shall exactly follow DMR. The records shall follow Good Manufacturing Practice with good traceability and document control.
In reality, the regulation does not require the specific tests or document format about documentation. These documents are required as a document evidence of product design and manufacturing quality to customer or 3rd party.
The art of manufacturing shall be based on each specific manufacturing process. Many guidance could be referred and searched on the website to meet the regulatory requirement.
Overall, the critical variable control of each specific manufacturing process and personal training are the key point to obtain a high-quality product. These documentations could be considered as a minimum requirement to prove product design and manufacturing quality.


Great and articulate delivery for medical device design record breakdown.