Medical Device- Process Validation in QMS
“Establish by objective evidence that a process consistently produces a result or product meeting its predetermined requirements.” – Process Validation This is... Read More
Medical Device- Design Control Documentation (DHF, DMR, DHR)
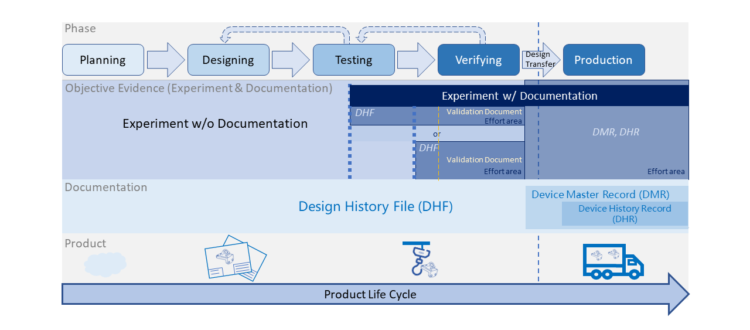
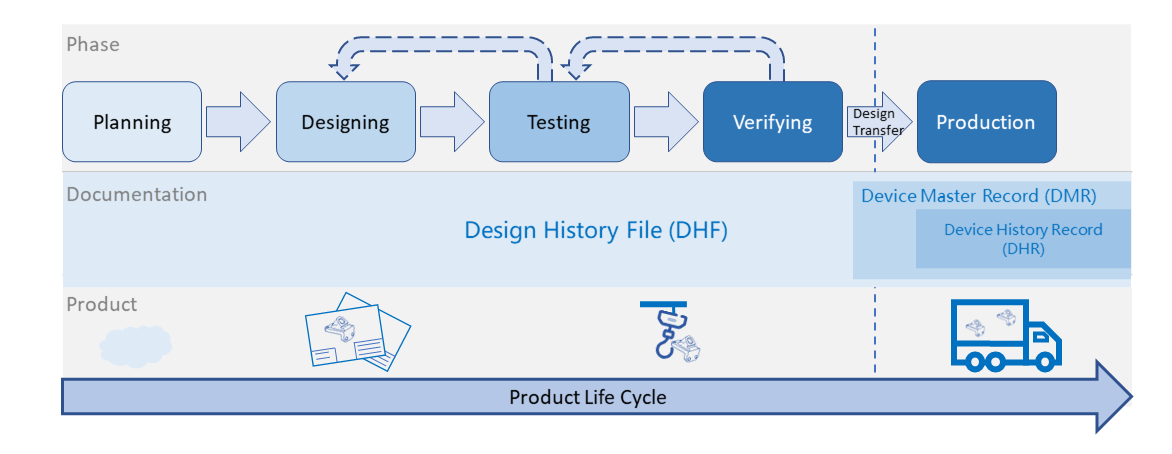
To launch a new product in medical device industry, many documents (e.g. design documents, verification documents, controlled documents, production records) are required... Read More