Recent Posts
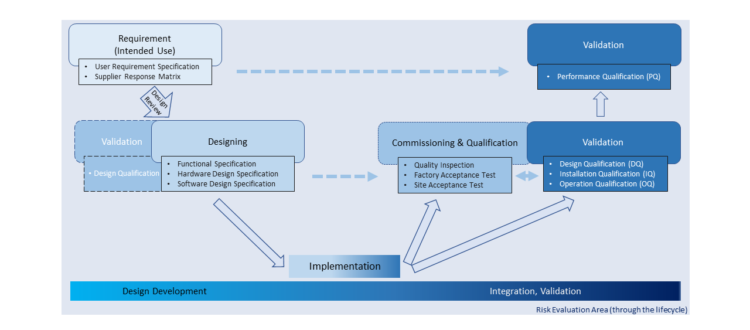
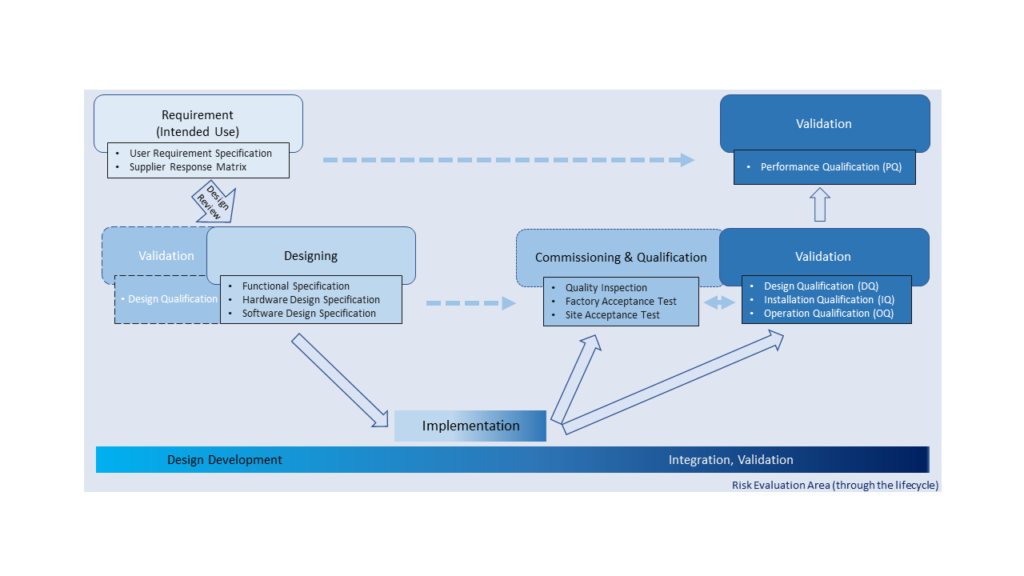
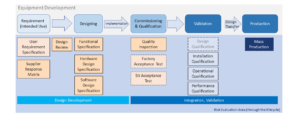
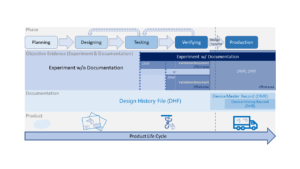
Basic Introduction of Equipment Development & Qualification
The objective of validation is to provide the evidence that the process can consistently and reproducibly produce parts... Read More
Medical Device- Process Validation in QMS
“Establish by objective evidence that a process consistently produces a result or product meeting its predetermined requirements.” –... Read More
Categories
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | ||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| 23 | 24 | 25 | 26 | 27 | 28 | |